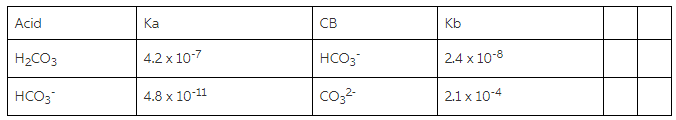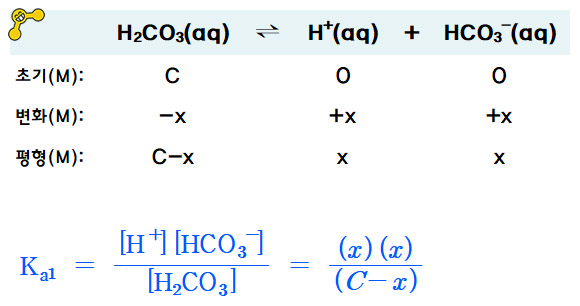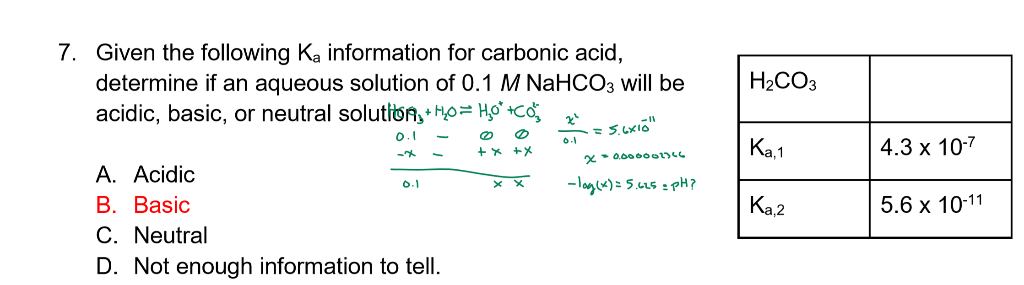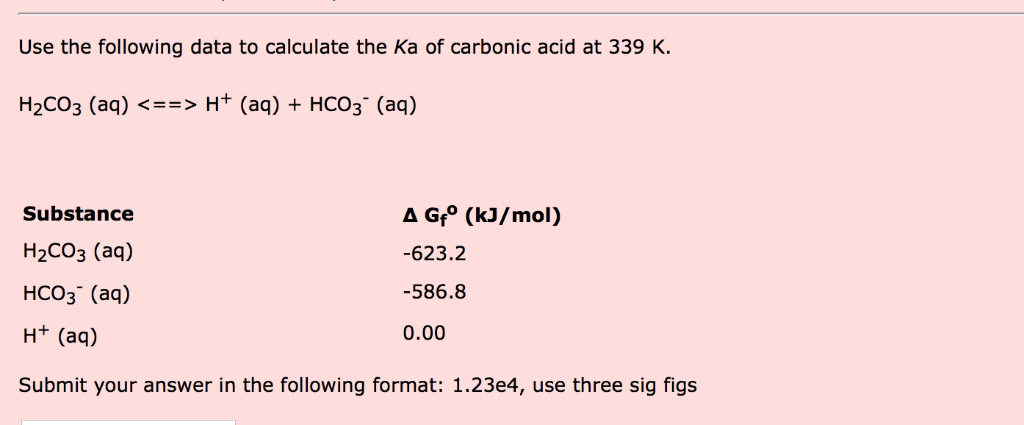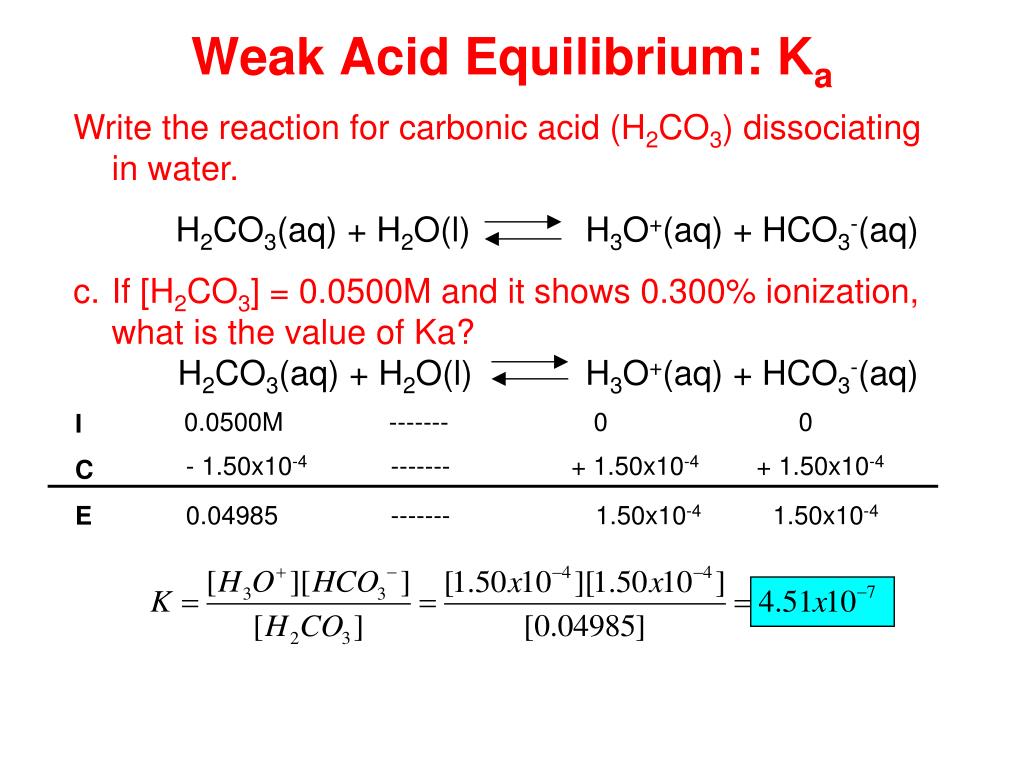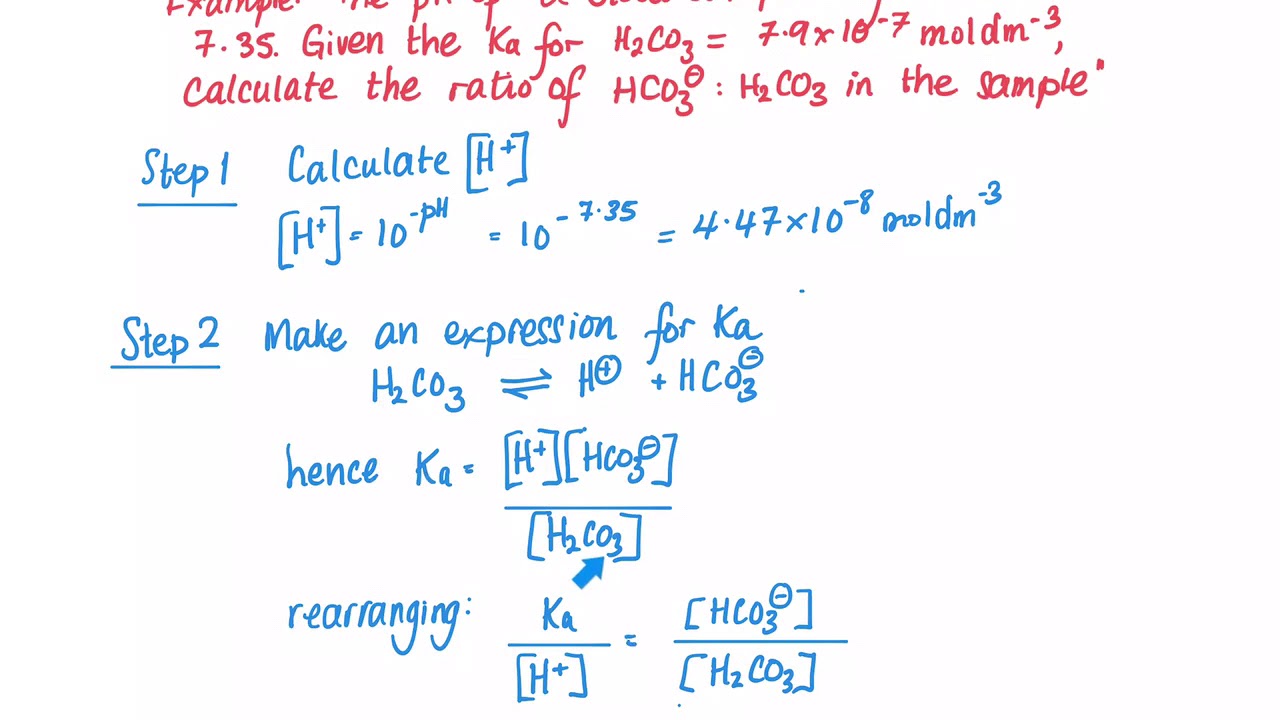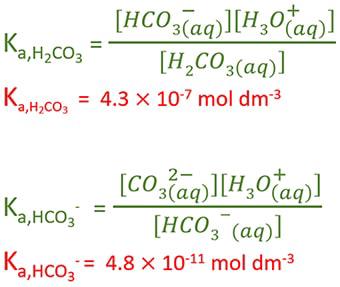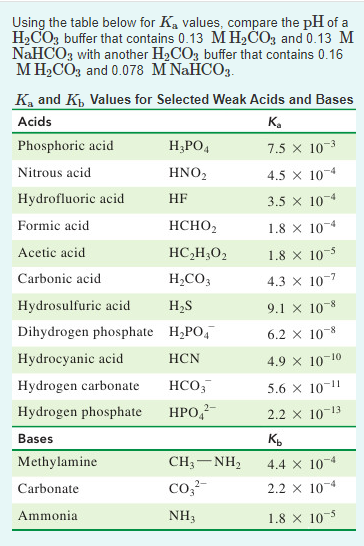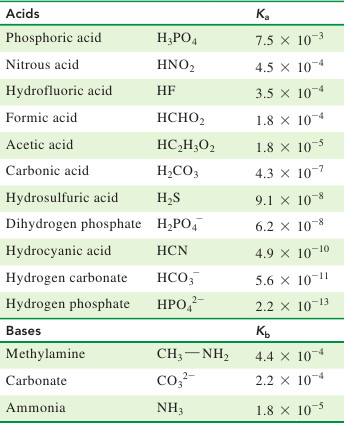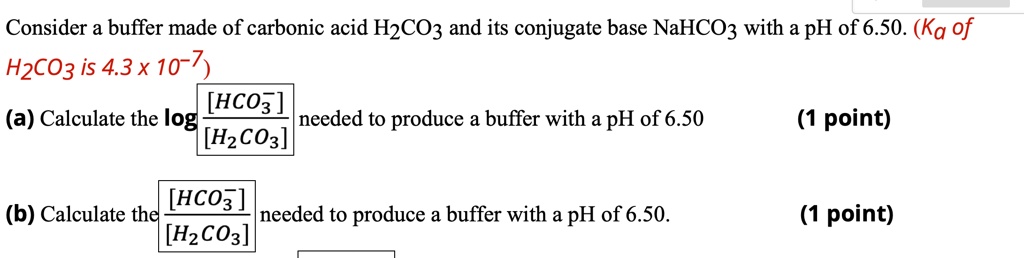
SOLVED: Consider a buffer made of carbonic acid HzCO3 and its conjugate base NaHCO3 with a pH of 6.50. (Ka of H2CO3 is 4.3x10-7) [HCO3 (a) Calculate the log needed to produce

Thermal stability of β-H2CO3 in the product mixture at 260 K, ∼400 mbar... | Download Scientific Diagram
Why is carbonic acid a weak acid even though it gets completely dissociated into H+ and CO3- ions? - Quora

The Ka of carbonic acid is 4.3 x 10-7. H2CO3 = H+ + HCO3 This means that H2co3 is a____. A.good - Brainly.com
![OneClass: For a solution of 1.4 M H2CO3 (Ka = 4.4 × 10-7), calculate: a) [H+] b) pH c) percent ioniz... OneClass: For a solution of 1.4 M H2CO3 (Ka = 4.4 × 10-7), calculate: a) [H+] b) pH c) percent ioniz...](https://prealliance-textbook-qa.oneclass.com/qa_images/homework_help/question/qa_images/130/13033912.png)
OneClass: For a solution of 1.4 M H2CO3 (Ka = 4.4 × 10-7), calculate: a) [H+] b) pH c) percent ioniz...

For carbonic acid the Ka1 = 4.30 × 10^-7 and the Ka2 = 5.62 × 10^-11. Calculate the pH of a 0.15 M solution of Na2CO3 :

Sejumlah H2CO3 (Ka = 4,3 x 1O-7) dicampurkan dengan larutan Ca(OH)2 membentuk larutan penyangga. Setelah - Brainly.co.id
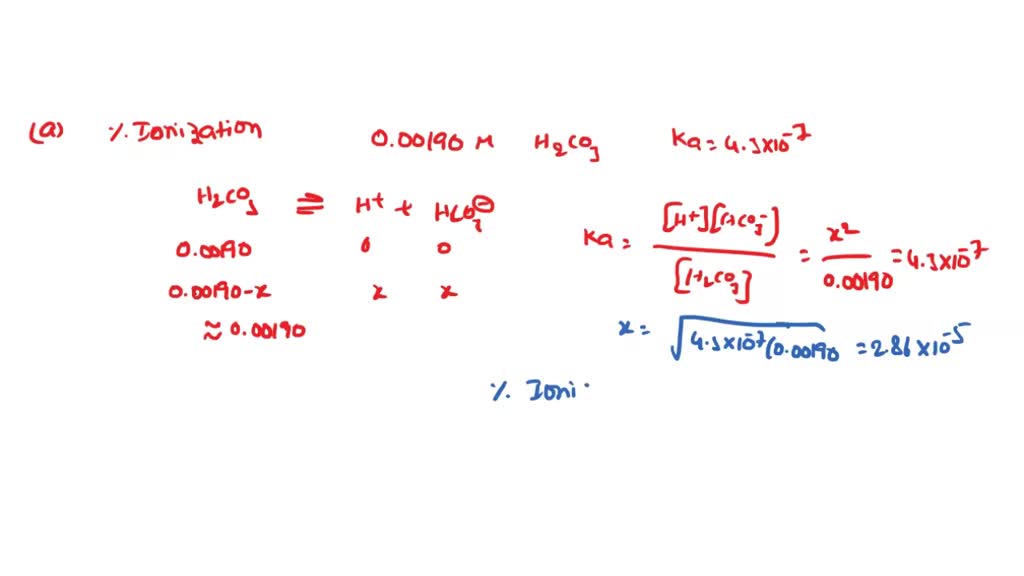
SOLVED: Calculate the percent ionization of carbonic acid (H2CO3) in solutions of each of the following concentrations (Ka = 4.3e-07.) (a) 0.281 M % (b) 0.366 M % (c) 0.641 M %