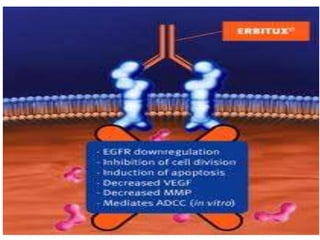
Bispecific antibodies for cancer therapy: the light at the end of the tunnel? - Abstract - Europe PMC

Wild-Type BRAF Is Required for Response to Panitumumab or Cetuximab in Metastatic Colorectal Cancer | Journal of Clinical Oncology

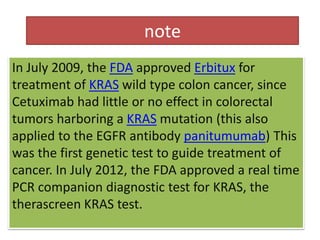
Wild-Type KRAS Is Required for Panitumumab Efficacy in Patients With Metastatic Colorectal Cancer | Journal of Clinical Oncology

QIAGEN Receives FDA Approval of therascreen® KRAS RGQ PCR Kit Paired with Second Colorectal Cancer Drug